Self-Administered Test Now in Early Testing Phase
BOSTON – A new clinical study being led by doctors at Massachusetts General Hospital (MGH), Brigham and Women’s Hospital (BWH), and Spaulding Rehabilitation Hospital could hold the key to unlocking early COVID-19 diagnoses. Researchers are pioneering a self-administered smell test that may detect the early stages of smell loss. This early awareness of exposure may trigger testing sooner and improve the overall accuracy of testing for COVID-19. Emerging research on patients infected with COVID-19 shows that self-reporting loss of sense of smell and taste are common early symptoms of the disease.
“There is so much we don’t know about COVID-19, but the research shows that loss of smell and taste play a prominent role in identifying possible patients with the virus,” said Dr. Mark Albers, an MGH neurologist specializing in memory and olfactory disorders and the principal investigator of the study. “If we can provide reliable self-administered tests to people and health care workers we may be able to slow the spread of the disease in the future and chart recovery of smell function, which may be helpful to determine when it is safe to reengage after having the COVID infection.”
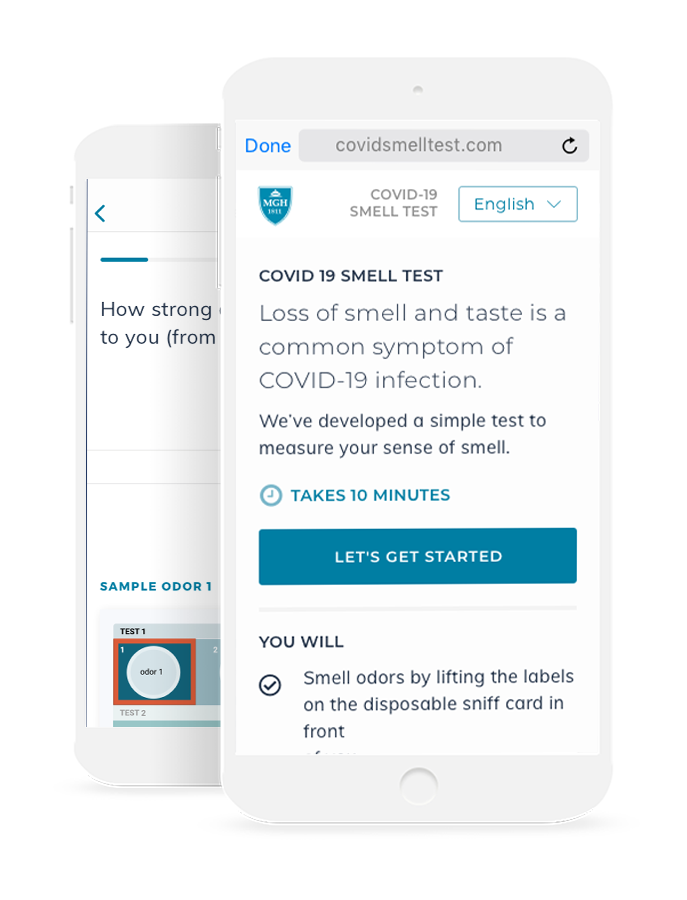
This objective test of smell function coupled with tracking of symptoms is self-administered by participants on their personal smart phone or home computer to reduce the risk of transmission of COVID-19. Responses about symptoms and the presented odors are entered into a new digital health app developed in collaboration with the study leaders.
The smell study is being created in collaboration with IFF, a leading innovator of taste, scent, and nutrition, who donated the scents from their Living Technology collection. MFR Samplings, in Argentina, provided their support with 500 cards for the pilot study while Arcade Beauty, an American company, contributed odor labels. ADK Group, a Boston-based app developer is assisting in getting the first prototype of the app running for the pilot phase.

“IFF has a long history of developing innovative solutions for a multitude of global challenges,” said Dr. Gregory Yep, IFF’s Chief Scientific and Sustainability Officer. “Our ongoing collaboration with Dr. Albers underscores our commitment to do more good for people and planet, and I hope our donation can help contribute to a solution for this pandemic.”
After receiving the small card in person or by mail, the test can be conducted on each patient’s own phone, tablet, or home computer. On the app, subjects will answer a series of questions about COVID symptoms and subjective loss of smell and taste.

“Technology has a huge role to play in the work to end this pandemic and provide answers to researchers,” said Dan Tatar, Founder and CEO of ADK Group. “ADK is excited to bring our expertise to this study and to help shed more light on the disease.”
Up to 400 patients at MGH, Brigham and Women’s Hospital and the Spaulding Rehabilitation Hospital will participate in this first round of testing. The Blavatnik Sensory Disorders Fund at Harvard Medical School is supporting the development of standalone apps that will enable longitudinal symptom tracking and smell testing.

About IFF
At IFF (NYSE:IFF) (Euronext Paris: IFF) (TASE: IFF), we’re using Uncommon Sense to create what the world needs. As a collective of unconventional thinkers and creators, we put science and artistry to work to create unique and unexpected scents, tastes, experiences and ingredients for the products our world craves. Learn more at iff.com, Twitter, Facebook, Instagram, and LinkedIn.
About ADK Group
ADK Group is a Boston-based digital agency and application development company with a unique culture that is rooted in philanthropy, technology, and adventure. We do work that matters, with like-minded people and partners. Learn more at www.adkgroup.com and LinkedIn.
Media Contact:
Matt Wilder
matt@wilderstrategies.com
617-504-1718